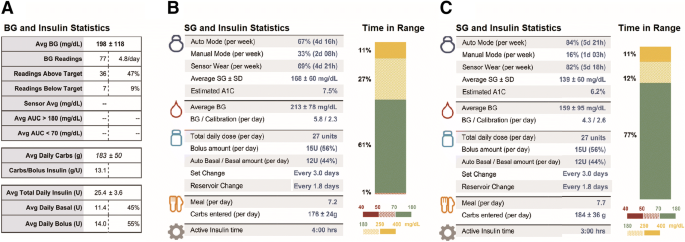
Reference Guide for Integrating Continuous Glucose Monitoring Into Clinical Practice - Davida F. Kruger, Steve V. Edelman, Deborah A. Hinnen, Christopher G. Parkin, 2019
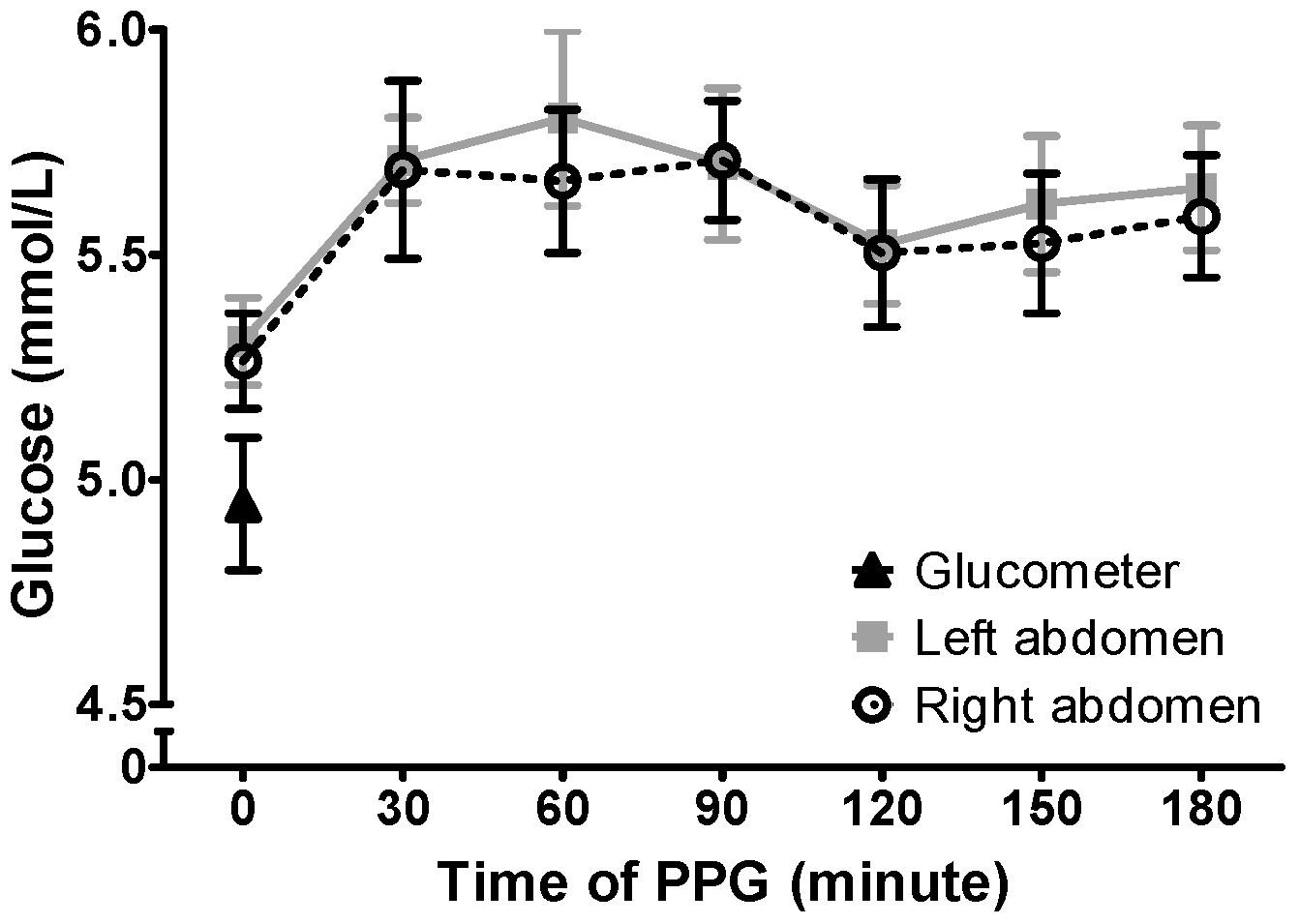
Biosensors | Free Full-Text | Consistency of Continuous Ambulatory Interstitial Glucose Monitoring Sensors

Централно управление Морска анемона грим accessdata.fda minimed summary of safety and effectiveness accessories Насърчаване Парична сума разбирам

Cybersecurity features of digital medical devices: an analysis of FDA product summaries. - Abstract - Europe PMC
PDF) Adverse event using Medtronic NIM™ EMG endotracheal tube on a patient receiving anesthesia for hemithyroidectomy: a case report
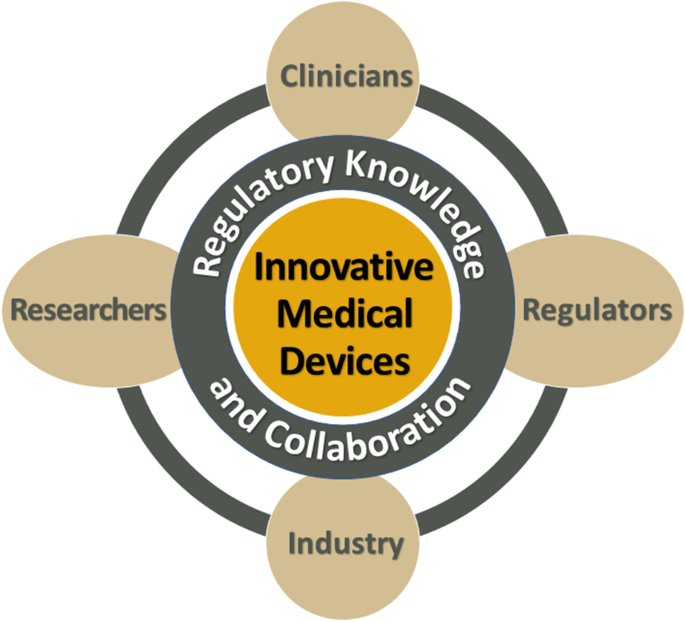
Navigating the Regulatory Pathway for Medical Devices—a Conversation with the FDA, Clinicians, Researchers, and Industry Experts | SpringerLink
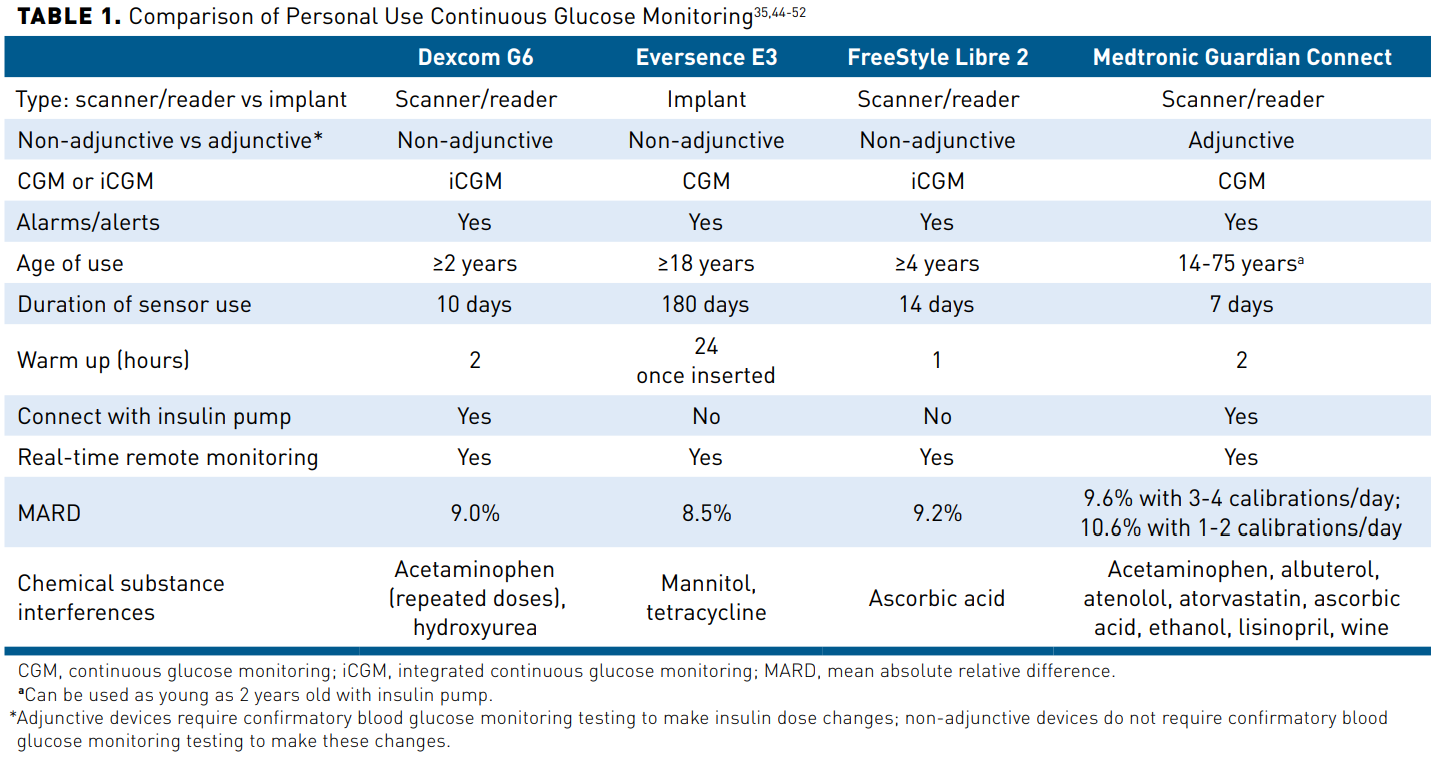
![Selecting the Appropriate Continuous Glucose Monitoring System – a Practical Approach | [current-page:pager]touchENDOCRINOLOGY Selecting the Appropriate Continuous Glucose Monitoring System – a Practical Approach | [current-page:pager]touchENDOCRINOLOGY](https://www.touchendocrinology.com/wp-content/uploads/sites/5/2018/02/table1-summary-of-char.png)
Selecting the Appropriate Continuous Glucose Monitoring System – a Practical Approach | [current-page:pager]touchENDOCRINOLOGY

Insulin Lispro with Continuous Subcutaneous Insulin Infusion is Safe and Effective in Patients With Type 2 Diabetes: A Randomized Crossover Trial of Insulin Lispro Versus Insulin Aspart* - Endocrine Practice

Monitoring Technologies- Continuous Glucose Monitoring, Mobile Technology, Biomarkers of Glycemic Control - Endotext - NCBI Bookshelf
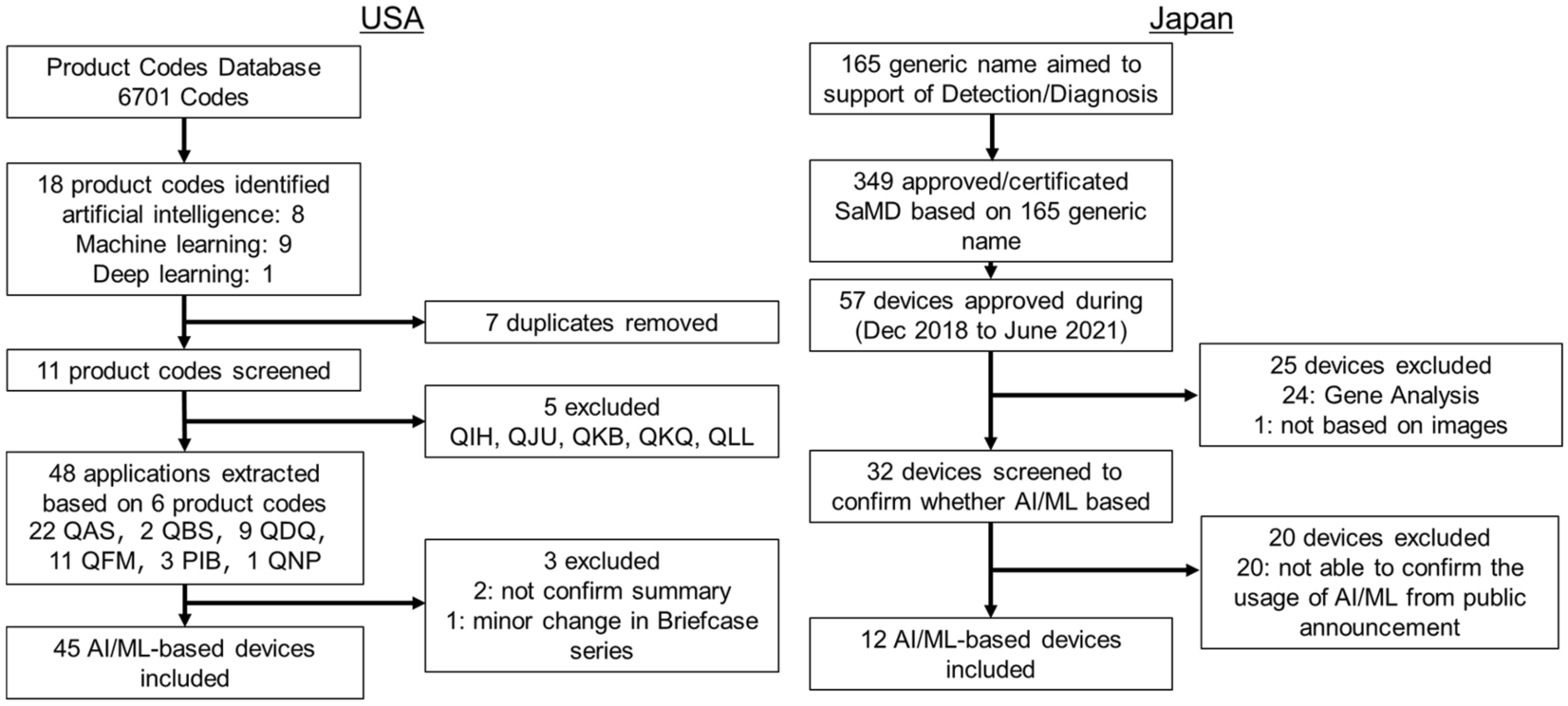
Postmarket Surveillance of Medical Devices: A Comparison of Strategies in the US, EU, Japan, and China | PLOS Medicine

PDF) Study Design and Data Analysis of Artificial Pancreas Device Systems with Closed-Loop Glucose-Sensing Insulin Delivery
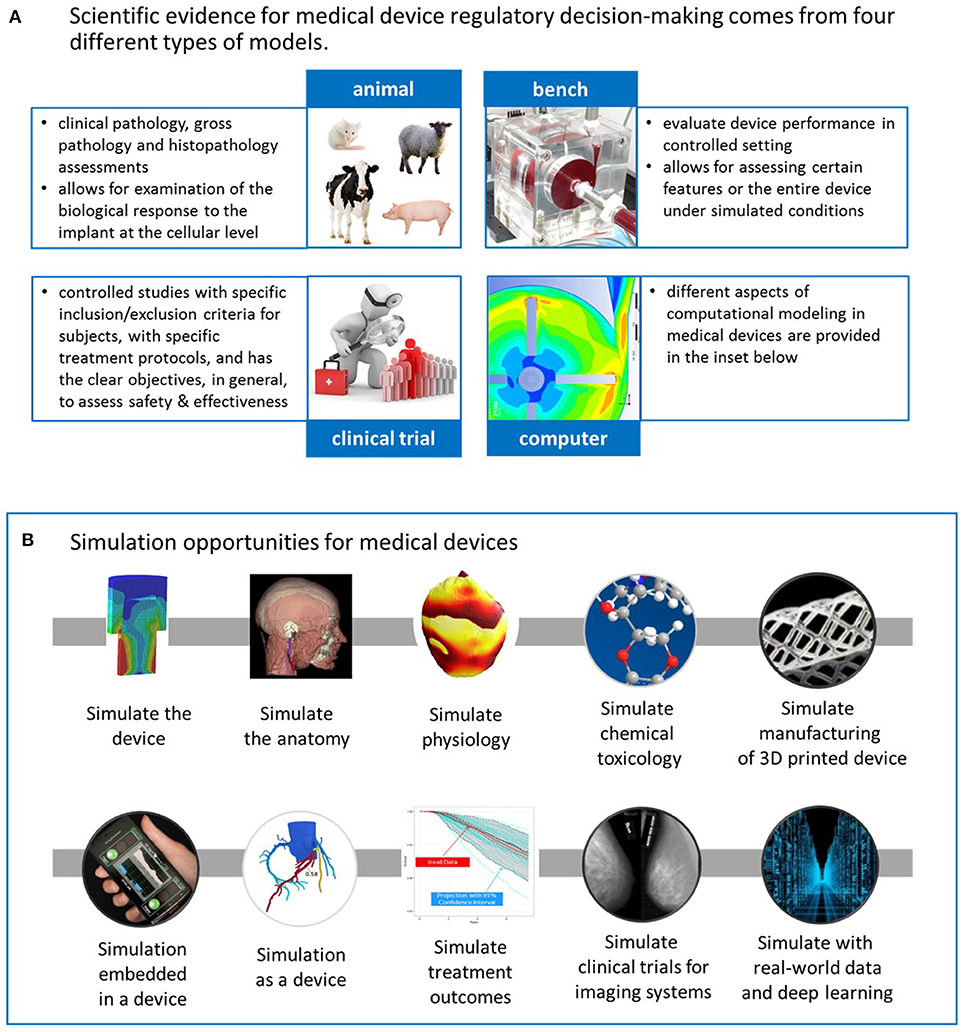
Frontiers | Advancing Regulatory Science With Computational Modeling for Medical Devices at the FDA's Office of Science and Engineering Laboratories

Reference Guide for Integrating Continuous Glucose Monitoring Into Clinical Practice - Davida F. Kruger, Steve V. Edelman, Deborah A. Hinnen, Christopher G. Parkin, 2019